When your dental practice is about to shut its doors for a long weekend or holiday, it’s easy to think that everything can simply pause — but your dental equipment doesn’t quite get that memo. One of the key backstage players that deserves attention is your dental unit waterlines.
Left untreated during downtime, these narrow tubing systems become havens for stagnation, biofilm formation, and microbial growth. Because you’re a dental hygiene-aware team, you know the stakes: your patients’ safety, your microbiological compliance, your peace of mind.
Here, we’ll walk through why dental waterlines are especially vulnerable during periods of non-use, what to do before and after the closure, and how the science of biofilm formation underscores the importance of vigilance.
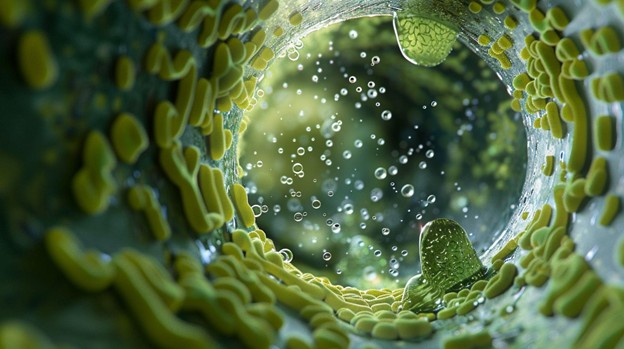
Why DUWLs Are Vulnerable During Closure
Your dental unit waterlines might seem harmless—after all, you aren’t treating patients—but behind the scenes there’s potential trouble. The tubing in dental unit waterlines is typically long and narrow, with a high surface-area-to-volume ratio, and during periods of idle time (such as an office closure) the water will sit. These conditions are exactly what microbial communities love.
Here are some of the mechanics of what’s going on:
- The tubing in waterlines may be 6 m or more of narrow diameter plastic, which gives a large internal surface relative to the water volume. That means microorganisms have more “real estate” to adhere to.
- Low flow or stagnation = limited shear force and minimal flushing, which helps early biofilm attach and spread.
- Biofilm formation is diverse: researchers found multiple microbial communities—including bacteria and fungi—in waterline biofilms, such as Pseudomonas, Acinetobacter, Candida, and even Legionella.
- Once a biofilm is established, it becomes more resistant to disinfectants and contaminates the water, putting your patients in danger of infection.
When the office closes and waterlines sit idle, you’re giving biofilm a head-start. That’s why it’s crucial to apply deliberate maintenance before and after the closure.
Practices Closed for Two Weeks or Less
Offices Using Daily Tablet Treatment (Citrisil Maintenance tablets):
Before the Closure/Holiday:
- Remove bottle from the manifold and empty out existing water.
- Fill bottles with fresh source water and add a new Citrisil Maintenance tablet just as you would for a standard operating day (follow the IFU).
- Connect bottle back to the manifold.
- Flush waterlines for 20-30 seconds each.
- Leave treated water in lines and bottles until you return. The treated water will continue to deliver a low-level antimicrobial to keep bacteria under control when not in use.
Upon Return from Closure/Holiday:
- Best practice is to start fresh. Empty bottles and refill with fresh source water and a new Citrisil Maintenance tablet just as you would for a standard operating day (follow the IFU).
- Before patient care resumes, flush the waterlines for at least 1 minute each. This removes stagnant water and the flow will help purge bacteria that may have accumulated.
- Begin patient care!
Offices Using Straw Treatment (Sterisil Straw):
Before the Closure/Holiday:
- Remove bottle from the manifold and empty out existing water.
- Fill bottles fully with fresh source water leaving the Sterisil Straw in place just as you would for a standard operating day (follow the IFU).
- Connect bottle back to the manifold.
- Flush waterlines for 20-30 seconds each.
- Leave treated water in lines and bottles until you return. The treated water will continue to deliver a low-level antimicrobial to keep bacteria under control when not in use.
Upon Return from Closure/Holiday:
- Best practice is to start fresh. Remove bottle from the manifold and empty out existing water.
- Fill bottles fully with fresh source water with the Sterisil Straw connected just as you would for a standard operating day and connect to the manifold (follow the IFU).
- Check that the Sterisil Straw is securely connected to the bottle pickup tube and pressure is set to the recommended PSI (40 PSI Sterisil Straw).
- Before patient care resumes, flush the waterlines for at least 1 minute each. This removes stagnant water and the flow will help purge bacteria that may have accumulated.
- Begin patient care!
Offices Without Dental Unit Bottles (Sterisil In-Line/Valved Cartridge):
Before the Closure/Holiday:
- These dental units do not have bottles and are directly plumbed into the municipal water supply and/or source water reservoir with either a Sterisil direct-feed treatment cartridge.
- Flush waterlines for 20-30 seconds each.
- Leave treated water in lines until you return. The treated water will continue to deliver a low-level antimicrobial to keep bacteria under control when not in use.
Upon Return from Closure/Holiday:
- Best practice is to start fresh. Before patient care resumes, flush the waterlines for at least 1 minute each. This removes stagnant water and the flow will help purge bacteria that may have accumulated.
- Since these dental units cannot be shocked routinely, it is highly recommended that all lines be tested prior to patient care.

The Science of Biofilm: Why This Matters
Understanding the “why” behind these actions empowers compliance and gives you credibility when discussing it with your team. Here’s a more detailed look at how biofilm formation works in dental unit waterlines and why downtime is a risk:
- Biofilm is a structured microbial community attached to a surface and embedded in a protective matrix that makes it very difficult to remove from the inner walls of waterlines once it’s formed.
- Pathogens frequently present in dental waterline biofilms include Pseudomonas aeruginosa, Legionella pneumophila, Acinetobacter, Candida, and others.
- Once a biofilm is established, it becomes more resistant to disinfectants and more likely to release microbial cells into the water stream. For example, a study found that even new chairs had heavy contamination before shock treatment—and after only one treatment some remained non-compliant.
- Key practical factors in contamination risk: age of the dental chair unit, number of patients treated, low water flow, periods of stagnation, location of anti-retraction valves or their failure.
Given this science, when you plan a closure you are essentially allowing stagnation (low flow) which supports biofilm attachment and growth—making your simple purge/flush + restart plan not just optional, but a critical infection-prevention measure.
Want-to-Know & Pitfalls to Avoid
- Closure length matters. A regular overnight or weekend closure may warrant the normal flush/purge. But longer periods may require additional precautions like shock treatments, bottle removal, or more intensive flushing.
- Don’t rely on “because we only had one patient that day.” Even small downtime allows detachment and reseeding of microbes; the internal walls don’t rest just because the chair does.
- Following the manufacturer’s Instructions For Use are crucial. Every dental unit model and every water-treatment product has specifics. For example, some require removing bottles, some specify “fill but idle flush,” some need line caps.
- Documenting is not just red-tape. It helps you demonstrate you followed recommended protocols in case of water-quality failure or regulatory review.
- Educate the team. Dental hygienists, assistants, and business staff should be aware that downtime is a risk point for dental unit waterline contamination—not just patient care time. Framing it as “pre-maintenance to keep our water safe and compliant” helps buy-in.
- Stay consistent. After reopening and restart, don’t slip into neglect mode. Biofilm doesn’t care if you think the line is “fresh”—regular maintenance is the only way to stay ahead of it.
In Summary
When your practice closes for a long weekend or holiday, your dental unit waterlines need attention—not just when you’re treating patients, but while you’re not. Because of the tubing geometry, low flow, and the nature of biofilm formation, periods of non-use create risk for stagnation and microbial accumulation.
By purging and flushing before closure, following manufacturer instructions, performing a thorough restart flush, and returning to your regular maintenance rhythm, you protect your patients, your team, and your compliance standing. And by understanding the underlying science of biofilm, you reinforce that this isn’t “extra work” — it’s essential risk management.